3d drawing of a water molecule
Geometry of Molecules
- Page ID
- 1991
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Agreement the molecular construction of a compound tin assist determine the polarity, reactivity, stage of affair, color, magnetism, equally well as the biological activity.
Introduction
To determine the shapes of molecules, nosotros must become acquainted with the Lewis electron dot construction. Although the Lewis theory does not determine the shapes of molecules, information technology is the beginning step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSPER) theory to determine the molecular geometry and the electron-grouping geometry.
To identify and take a complete description of the three-dimensional shape of a molecule, we need to know likewise learn nearly state the bond angle also. Lewis Electron Dot Structures play crucial role in determining the geometry of molecules because it helps us identify the valence electrons. To acquire how to draw a Lewis electron dot structure click the link higher up.
Valence-Beat out Electron-Pair Repulsion Theory
Now that we have a background in the Lewis electron dot construction we can use it to locate the the valence electrons of the middle atom. The valence-shell electron-pair repulsion (VSEPR) theory states that electron pairs repel each other whether or not they are in bond pairs or in lone pairs. Thus, electron pairs will spread themselves as far from each other as possible to minimize repulsion. VSEPR focuses non but on electron pairs, but it also focus on electron groups as a whole. An electron group can be an electron pair, a alone pair, a single unpaired electron, a double bond or a triple bail on the heart atom. Using the VSEPR theory, the electron bail pairs and lone pairs on the center cantlet will assist us predict the shape of a molecule.
The shape of a molecule is determined by the location of the nuclei and its electrons. The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction. Thus, the molecule's shape reflects its equilibrium state in which information technology has the everyman possible energy in the system. Although VSEPR theory predicts the distribution of the electrons, nosotros have to take in consideration of the bodily determinant of the molecular shape. We dissever this into ii categories, the electron-grouping geometry and the molecular geometry.
Electron-group geometry is determined past the number of electron groups.
| Number of electron groups | Name of electron grouping geometry |
|---|---|
| 2 | linear |
| iii | trigonal-planar |
| 4 | tetrahedral |
| 5 | trigonal-bipyramidal |
| 6 | octahedral |
Molecular geometry, on the other hand, depends on non but on the number of electron groups, but also on the number of lone pairs. When the electron groups are all bond pairs, they are named exactly like the electron-group geometry. Come across the chart below for more information on how they are named depending on the number of solitary pairs the molecule has.
VSEPR Notation
As stated higher up, molecular geometry and electron-grouping geometry are the aforementioned when in that location are no solitary pairs. The VSEPR note for these molecules are AXn . "A" represents the primal atom and n represents the number of bonds with the central cantlet. When alone pairs are present, the letter Ex is added. The ten represents the number of alone pairs present in the molecule. For case, a molecule with two bond pairs and ii lonely pairs would take this notation: AXiiE2 .
| Number of Electron Groups | Electron-Grouping Geometry | Number of Alone Pairs | VSEPR Notation | Molecular Geometry | Ideal Bond Angles | Examples |
|---|---|---|---|---|---|---|
| 2 | linear | 1 | AXii | 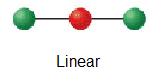 | 180° | BeH2 |
| 3 | trigonal-planar | 0 | AX3 | 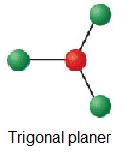 | 120° | CO3 2 - |
| 1 | AX2E | 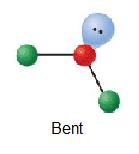 | 120° | O3 | ||
| 4 | tetrahedral | 0 | AX4 | Tetrahedral | 109.5° | S04 2 - |
| 1 | AX3E | 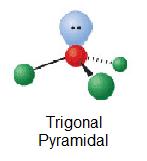 | 109.5° | H3O+ | ||
| 2 | AX2Eii | 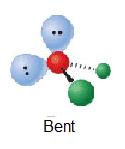 | 109.v° | H2O | ||
| 5 | trigonal-bipyramidal | 0 | AX5 | 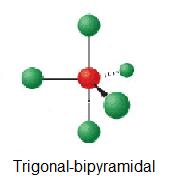 | 90°, 120° | PF5 |
| i | AXfourEb | 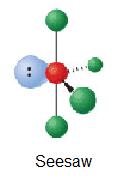 | xc°, 120° | TeCliv | ||
| ii | AX3East2 | 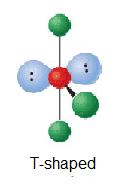 | ninety° | ClF3 | ||
| iii | AX2E3 | 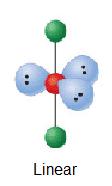 | 180° | Iiii - | ||
| 6 | octahedral | 0 | AXhalf dozen | octahedral | xc° | PF6 - |
| ane | AXvEast | 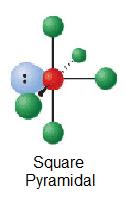 | 90° | SbCl5 ii - | ||
| 2 | AX4Eii | 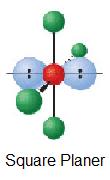 | 90° | ICl4 - |
Instance \(\PageIndex{1}\):
Lets attempt determining the geometric structures of H2O and CO2. So starting off by drawing the Lewis structure:
H2O:
H2o has four electron groups so it falls under tetrahedral for the electron-group geometry. The four electron groups are the 2 single bonds to Hydrogen and the two alone pairs of Oxygen. Since water has two lone pairs it's molecular shape is bent. Co-ordinate to the VSEPR theory, the electrons desire to minimize repulsion, so as a outcome, the solitary pairs are next from each other.
COtwo:
Carbon dioxide has two electron groups and no solitary pairs. Carbon dioxide is therefore linear in electron-group geometry and in molecular geometry. The shape of CO2 is linear because there are no lone pairs affecting the orientation of the molecule. Therefore, the linear orientation minimizes the repulsion forces.
Molecules with More than One Central Atom
The VSEPR theory not only applies to one central atom, but it applies to molecules with more than than one central atom. We take in account the geometric distribution of the terminal atoms effectually each central atom. For the concluding description, we combine the separate description of each atom. In other words, we take long chain molecules and break it down into pieces. Each piece will form a particular shape. Follow the instance provided beneath:
Butane is CfourH10. C-C-C-C is the simplified structural formula where the Hydrogens (not shown) are implied to take single bonds to Carbon. Y'all can view a better structural formula of butane at en.Wikipedia.org/wiki/File:Butane-2D-flat.png
If we interruption downwardly each Carbon, the central atoms, into pieces, we tin can make up one's mind the relative shape of each section. Let'due south beginning with the leftmost side. Nosotros run into that C has three single bonds to 2 Hydrogens and 1 single bond to Carbon. That means that nosotros have 4 electron groups. By checking the geometry of molecules chart in a higher place, nosotros have a tetrahedral shape. At present, we move on to the adjacent Carbon. This Carbon has ii single bonds to 2 Carbons and two single bonds to ii Hydrogens. Again, we have 4 electron groups which effect in a tetrahedral. Continuing this trend, we take another tetrahedral with unmarried bonds attached to Hydrogen and Carbon atoms. As for the rightmost Carbon, nosotros likewise have a tetrahedral where Carbon binds with 1 Carbon and 3 Hydrogens.
Permit me recap. We took a look at butane provided by the wonderful Wikipedia link. We, then, broke the molecule into parts. We did this by looking at a particular central atom. In this instance, we have 4 key atoms, all Carbon. By breaking the molecule into 4 parts (each role looks at 1 of the 4 Carbons), nosotros determine how many electron groups there are and detect out the shapes.
Nosotros aren't done, yet! We demand to decide if there are any lonely pairs because we only looked at bonds. Remember that electron groups include alone pairs! Butane doesn't have any lone pairs. Hence, we have 4 tetrahedrals. Now, what are we going to practise with 4 tetrahedrals? Well, we want to optimize the bail bending of each central atom attached to each other. This is due to the electrons that are shared are more than likely to repel each other. With four tetrahedrals, the shape of the molecule looks like this: en.Wikipedia.org/wiki/File:Butane-3D-balls.png. That ways that if we expect dorsum at every individual tetrahedral, we match the key Carbon with the Carbon information technology's bonded to.
Bond Angles
Bail angles also contribute to the shape of a molecule. Bond angles are the angles between next lines representing bonds. The bond angle tin assistance differentiate between linear, trigonal planar, tetraheral, trigonal-bipyramidal, and octahedral. The ideal bail angles are the angles that demonstrate the maximum angle where information technology would minimize repulsion, thus verifying the VSEPR theory.
Essentially, bond angles is telling us that electrons don't similar to be near each other. Electrons are negative. Two negatives don't concenter. Let's create an analogy. Mostly, a negative person is seen equally bad or mean and you don't want to talk to a negative person. One negative person is bad enough, merely if you have ii put together...that'south just horrible. The ii negative people will exist mean towards each other and they won't similar each other. So, they will exist far away from each other. We tin can utilize this idea to electrons. Electrons are akin in charge and volition repel each other. The farthest fashion they can get away from each other is through angles. At present, let's refer back to tetrahedrals. Why is information technology that 90 degrees does not work? Well, if nosotros draw out a tetrahedral on a 2-D plane, then we get 90 degrees. Withal, nosotros live in a iii-D world. To visualize this, think almost movies. Movies in 3D pop out at u.s.. Before, we see movies that are just on the screen and that'due south skilful. What'southward better? 3D or 2D? For bond angles, 3D is better. Therefore, tetrahedrals accept a bail angle of 109.v degrees. How scientists got that number was through experiments, simply we don't need to know too much detail because that is not described in the textbook or lecture.
Using the example above, we would add that H2O has a bond bending of 109.5° and CO2 would take a bond angle of 180°.
Steps Used to Find the Shape of the Molecule
To sum upward in that location are four simple steps to apply the VSEPR theory.
- Draw the Lewis Construction.
- Count the number of electron groups and place them equally bond pairs of electron groups or lone pairs of electrons. Recall electron groups include non but bonds, but also lone pairs!
- Name the electron-grouping geometry. (State whether it is linear, trigonal-planar, tetrahedral, trigonal-bipyramidal, or octahedral.)
- Looking at the positions of other atomic nuclei around the cardinal make up one's mind the molecular geometry. (Meet how many lone pairs there are.)
Dipole Moments
A molecule is polar when the electrons are not distributed equally and the molecule has two poles. The more electronegative end of the molecule is the negative end and the less electronegative end is the positive end. A common instance is HCl. Using the capital sigma + or - equally a symbol to evidence the the positive end and the negative cease we can describe the cyberspace dipole. And so sigma + would exist on the hydrogen atom and sigma - would be on the Chlorine atom. Using the cantankerous bow pointer shown below we can show that information technology has a net dipole. The net dipole is the measurable, which is called the dipole moment. Dipole moment is equal to the product of the partial charge and the distance. The equation for dipole moment is every bit follows.
\[ \mu = \delta \times d\]
with
- µ = dipole moment ( debye )
- δ = fractional charge (C)
- d = altitude (m)
The units for dipole is expressed in debye which is also known as Coulombs x meter (C 10 k)
Instance of a Dipole
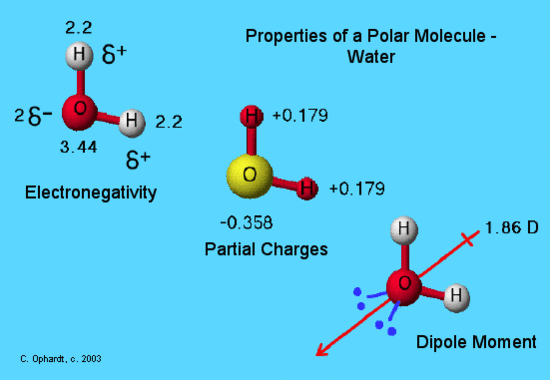
The cantankerous base arrow demonstrates the net dipole.
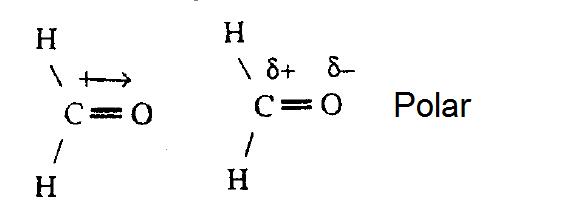
On the cross-base of operations arrow, the cross represents the positive charge and the arrow represents the negative charge.
Here's another way to determine dipole moments. Nosotros need to comprehend electronegativity which is abbreviated EN. What is EN? Well, EN is how much an element really wants an electron. Think nearly basketball and how two players pass the ball to each other. Each histrion represent an element and the ball represents the electron. Allow'southward say one player is a ball hog. The player that is the ball squealer is more electronegative because he or she wants the ball more.
Hither is a link that has all the EN listed: world wide web.green-planet-solar-energy...electroneg.gif
What if we are not given EN? Luckily, there is a trend in the periodic tabular array for EN. From bottom to the tiptop, EN will increase. From left to correct, EN will increment. The most electronegative element is Flourine with 4.0.
Now, we are set to apply EN to determine whether or not molecules are polar. We look back at the motion picture of H2O above. The EN is given. What practice we exercise with all the EN? Nosotros compare the EN between each bond. Oxygen has a greater EN than Hydrogen. Therefore, we can draw a cross bow pointer towards Oxygen. We have ii arrows because Oxygen is bonded to ii Hydrogens. Since both arrows point toward Oxygen, we can say that there is a net EN. We added the arrows that point to Oxygen and nosotros end upwards with a new, bigger arrow. This is examplified in the picture to a higher place. If arrows are fatigued abroad from each other like <--- and --->, so we are more than likely to have no net EN because the molecule is symmetrical. Refer back to the Lewis dot diagram of CO2. The shape is linear and the EN arrows point towards Oxygen. The arrows are reverse of each other and have the same EN difference. Therefore, nosotros have no net accuse and the molecule is not-polar.
Summary of Dipole Moments
To recap, when a molecule is polar it means that the electron is non distributed evenly and there is a difference in the electronegativity of the atoms. If a molecule is polar, it means that it had a internet dipole which results in having a dipole moment.
Determining Polarity
Is it polar? There are three means to get most determining whether a molecule is polar or not.
A. If the molecule has a net dipole, then it is polar.
B. If the structure is symmetric, then information technology is non-polar
C. In that location are three rules to this part:
1. When there are no lonely pairs on the center cantlet, then the molecule is not-polar
ii. If it is linear or square planar, then information technology is non-polar. (This rule is more of import than dominion 1, so it overrules it because it has lone pairs.)
3. If it has dissimilar last atoms, and then it is polar. (This dominion overrules rule 1 and two because it is more important.)
References
- Petrucci, Ralph H., William South. Harwood, F. Geoffrey Herring, & Jeffry D. Madura, General Chemistry, Principles and Modernistic Appplications Ninth Edition, Upper Saddle River, New Jersey
- Tetrahedrality" and the Relationship between Commonage Construction and Radial Distribution Functions in Liquid H2o P. E. Mason and J. West. Brady J. Phys. Chem. B;2007
- Inverted geometries at carbon Kenneth B. Wiberg Acc. Chem. Res.; 1984
- "Molecular Geometries." Chemistry Foundations and Applications. Volume 3. Farmington, MI:Lagowski, J.J., 2004.
Problems
Function I
Draw the Lewis Construction and name the shape of each compound. Also decide the polarity and whether or not information technology has a dipole moment.
- HClO3
- Thenthree
- PClfour
- C2H4
- SnCl3 -
Part II
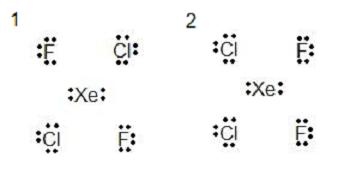
Proper name the shape and determine whether they are polar or non-polar.
Solutions
Part I
1.
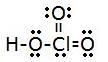
- Full # of electrons: 1+(3x6)+7=26
- electron group geometry: tetrahedral
- molecular: trigonal pyramidal
- platonic angle: 109.5°
- polar, has a dipole moment
2.
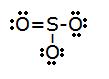
- Full # of electrons: (3x6)+half dozen=24
- electronic group geometry: trigonal planar
- molecular geometry: trigonal planar
- ideal bending: 120°
- polar, has a dipole moment
three.
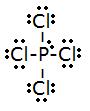
- Total # of electrons: (4x4)+5=19
- electronic group geometry: trigonal-bi-pyramidal
- molecular geometry: seesaw
- ideal angle: 90°, 120°
- polar, has a dipole moment
four.
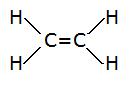
- Total # of electrons: (1x4)+(4x2)=12
- electronic group geometry: trigonal planar
- molecular geometry: trigonal planar
- ideal angle: 120°
- non-polar, does non take a dipole moment
5.
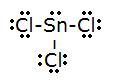
- Total # of electrons: (7x3)+four=26
- electronic group geometry: tetrahedral
- molecular geometry: trigonal pyramidal
- ideal angle: 109.5°
- polar, has a dipole moment.
Role II
1. electron grouping geometry: octahedral
molecular geometry: foursquare planar
non polar because information technology is symmetrical
ii. electron group geometry: octahedral
molecular geometry: square planar
polar because it is non symmetrical
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Lewis_Theory_of_Bonding/Geometry_of_Molecules
0 Response to "3d drawing of a water molecule"
Post a Comment